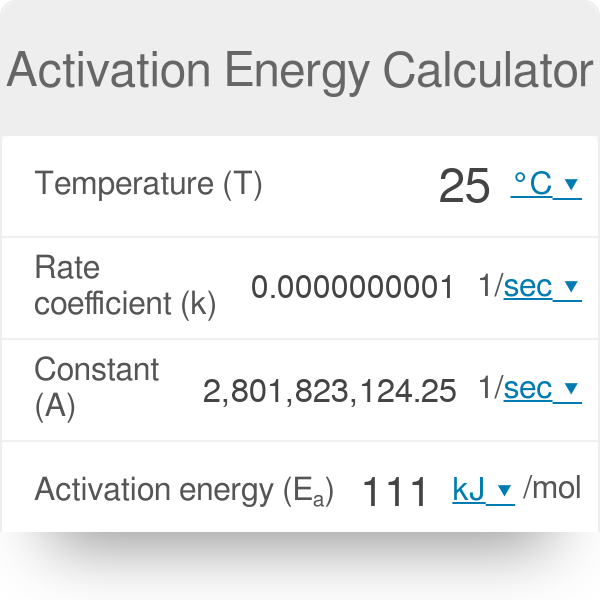
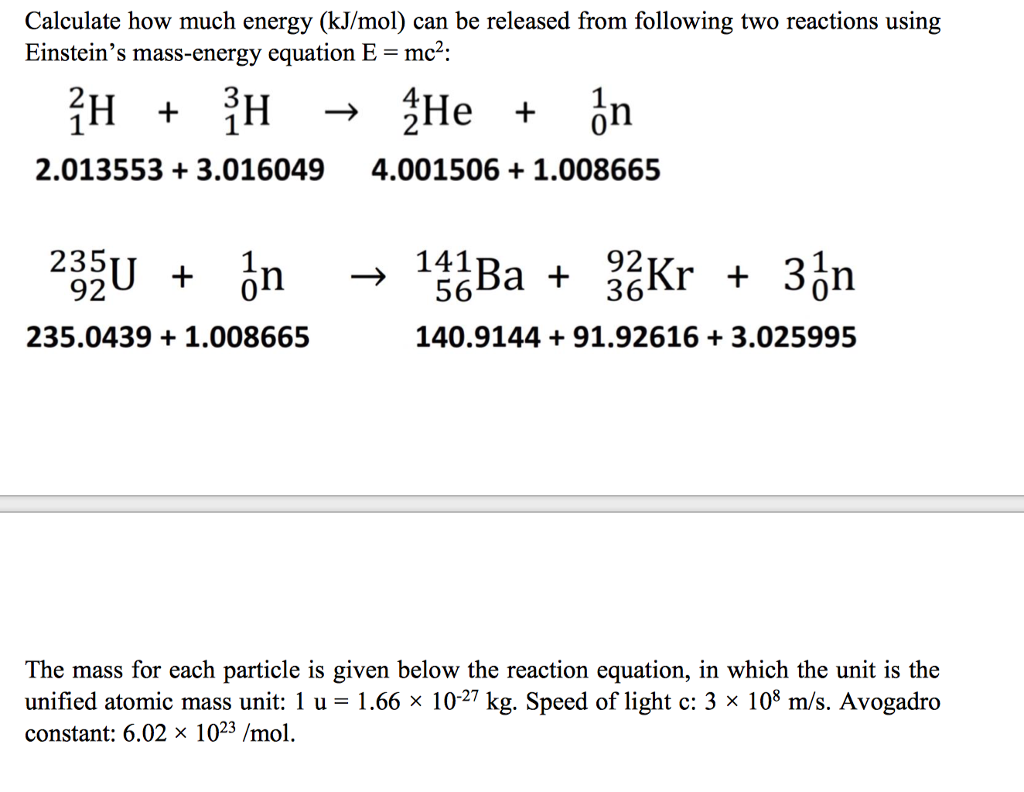
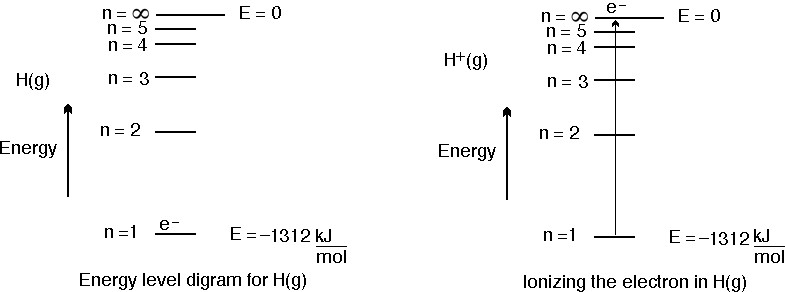
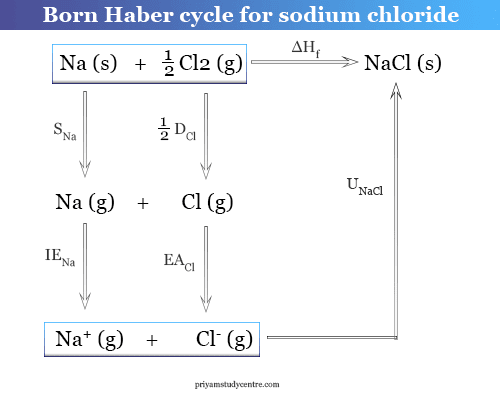
SOLVED:(II) Binding energies are often measured experimentally in kcal per mole, and then the binding energy in eV per molecule is calculated from that result. What is the conversion factor in going
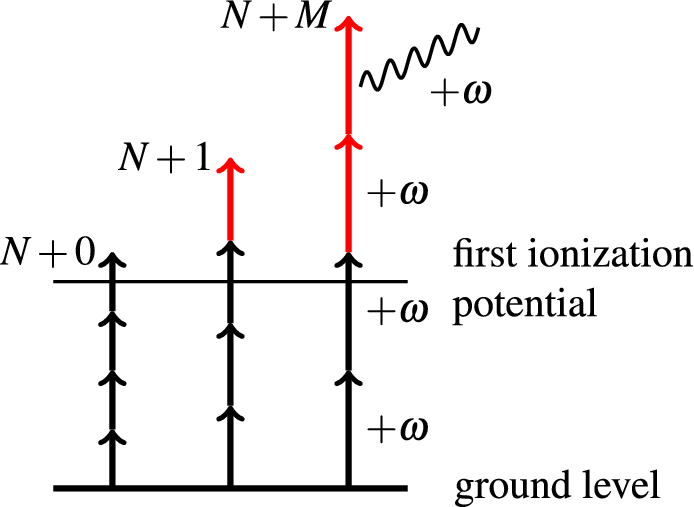
Multi-photon above threshold ionization of multi-electron atoms and molecules using the R-matrix approach | Scientific Reports

Calculate the wavelength of light required to break the bond between two chlorine atoms in a chlorine molecule. The Cl - Cl bond energy is 243 kJmol^-1. (h = 6.6 × 10^-34

density functional theory - How to calculate the enthalpy of formation? - Matter Modeling Stack Exchange

Calculate the energy in kilojoules per mole of electronic charge accelerated by a potantial of `... - YouTube